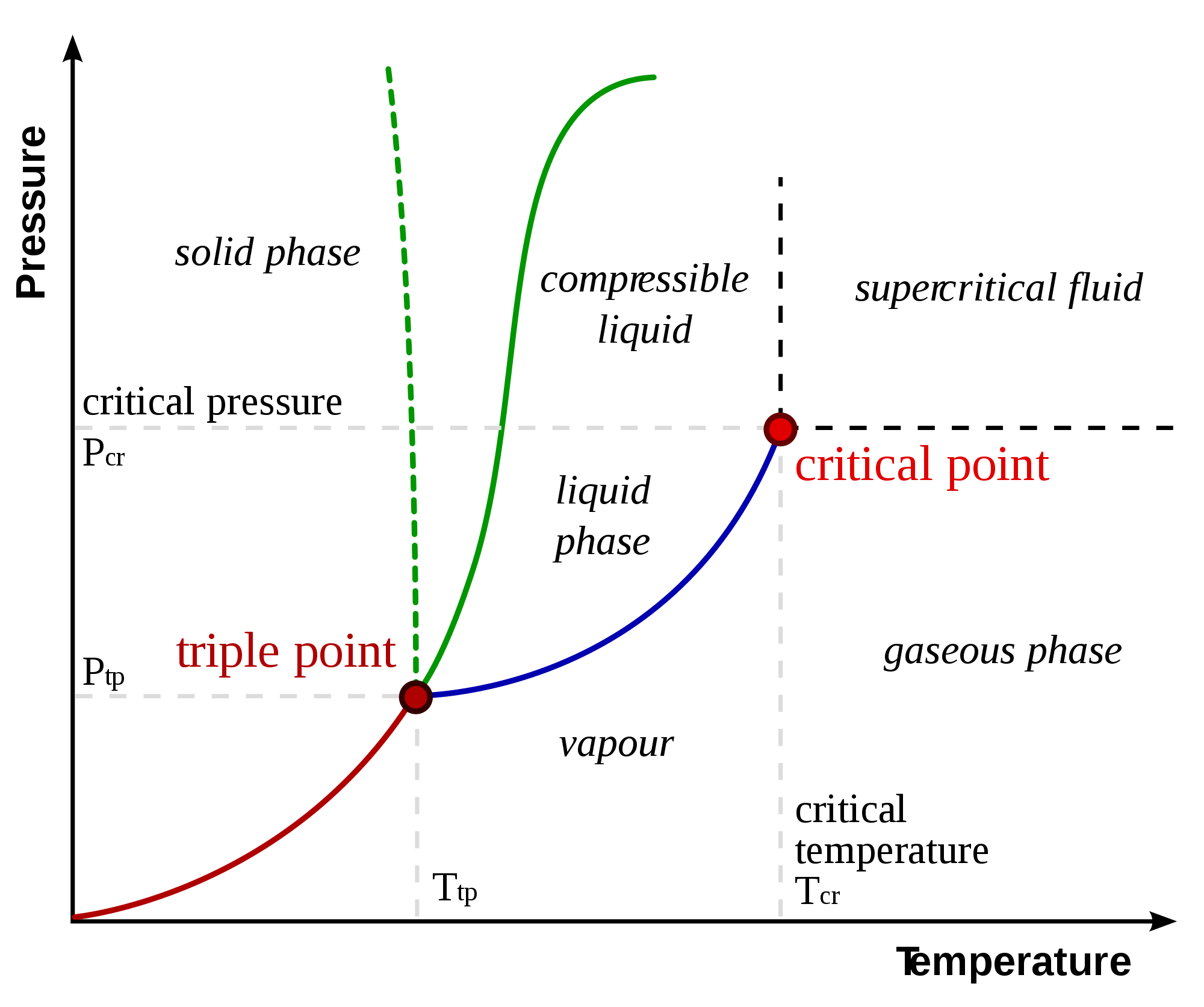
Describe The Region Where A Solid-Liquid Equilibrium Exists . a pure substance may exist in any of the three phases: The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. to be able to identify the triple point, the critical point, and four regions: consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. When its temperature or pressure. Solid, liquid, and vapour, at certain temperatures and pressures. Low temperature and high pressure favor solid.
from byjus.com
Solid, liquid, gas, and a supercritical fluid. to be able to identify the triple point, the critical point, and four regions: When its temperature or pressure. The state exhibited by a given. a pure substance may exist in any of the three phases: Low temperature and high pressure favor solid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Solid, liquid, and vapour, at certain temperatures and pressures.
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law
Describe The Region Where A Solid-Liquid Equilibrium Exists a pure substance may exist in any of the three phases: Solid, liquid, and vapour, at certain temperatures and pressures. Solid, liquid, gas, and a supercritical fluid. a pure substance may exist in any of the three phases: consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. to be able to identify the triple point, the critical point, and four regions: When its temperature or pressure. Low temperature and high pressure favor solid. The state exhibited by a given.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500701 Describe The Region Where A Solid-Liquid Equilibrium Exists a pure substance may exist in any of the three phases: to be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Low temperature and high pressure favor solid. The state exhibited by a given. consider an equilibrium between a crystalline salt (or other kind of ionic. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.youtube.com
SolidLiquid Equilibrium YouTube Describe The Region Where A Solid-Liquid Equilibrium Exists to be able to identify the triple point, the critical point, and four regions: The state exhibited by a given. Solid, liquid, and vapour, at certain temperatures and pressures. a pure substance may exist in any of the three phases: Solid, liquid, gas, and a supercritical fluid. When its temperature or pressure. consider an equilibrium between a. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Describe The Region Where A Solid-Liquid Equilibrium Exists to be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Solid, liquid, and vapour, at certain temperatures and pressures. Low temperature and high pressure favor solid. When its temperature. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.britannica.com
phase Definition & Facts Britannica Describe The Region Where A Solid-Liquid Equilibrium Exists Low temperature and high pressure favor solid. Solid, liquid, and vapour, at certain temperatures and pressures. to be able to identify the triple point, the critical point, and four regions: The state exhibited by a given. a pure substance may exist in any of the three phases: When its temperature or pressure. Solid, liquid, gas, and a supercritical. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.numerade.com
SOLVED Consider the phase diagram in the figure below, which Describe The Region Where A Solid-Liquid Equilibrium Exists Low temperature and high pressure favor solid. The state exhibited by a given. a pure substance may exist in any of the three phases: consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Solid, liquid, gas, and a supercritical fluid. to be able to identify the triple point,. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Describe The Region Where A Solid-Liquid Equilibrium Exists a pure substance may exist in any of the three phases: Solid, liquid, and vapour, at certain temperatures and pressures. to be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Low temperature and high pressure favor solid. When its temperature or pressure. The state exhibited by a. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From slideplayer.com
Chapter 11 Intermolecular Forces, Liquids, and Solids ppt download Describe The Region Where A Solid-Liquid Equilibrium Exists to be able to identify the triple point, the critical point, and four regions: Solid, liquid, and vapour, at certain temperatures and pressures. The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Low temperature and high. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Describe The Region Where A Solid-Liquid Equilibrium Exists The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Low temperature and high pressure favor solid. When its temperature or pressure. Solid, liquid, and vapour, at certain temperatures and pressures. to be able to identify the. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.numerade.com
SOLVED 4 Consider the phase diagram in the figure below, which Describe The Region Where A Solid-Liquid Equilibrium Exists The state exhibited by a given. Low temperature and high pressure favor solid. Solid, liquid, gas, and a supercritical fluid. a pure substance may exist in any of the three phases: Solid, liquid, and vapour, at certain temperatures and pressures. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the.. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Describe The Region Where A Solid-Liquid Equilibrium Exists The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. Low temperature and high pressure favor solid. Solid, liquid, and vapour, at certain temperatures and pressures. to be able to identify the triple point, the critical point, and four regions: a pure substance may exist in any of the three phases: When its temperature or. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Describe The Region Where A Solid-Liquid Equilibrium Exists consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Low temperature and high pressure favor solid. The state exhibited by a given. to be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Solid, liquid, and vapour, at certain. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From unistudium.unipg.it
Phase Diagrams Describe The Region Where A Solid-Liquid Equilibrium Exists The state exhibited by a given. Solid, liquid, and vapour, at certain temperatures and pressures. Low temperature and high pressure favor solid. When its temperature or pressure. to be able to identify the triple point, the critical point, and four regions: a pure substance may exist in any of the three phases: consider an equilibrium between a. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.slideserve.com
PPT Solid Liquid Phase Diagrams PowerPoint Presentation, free Describe The Region Where A Solid-Liquid Equilibrium Exists consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Low temperature and high pressure favor solid. Solid, liquid, and vapour, at certain temperatures and pressures. When its temperature or pressure. to be able to identify the triple point, the critical point, and four regions: The state exhibited by a. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From courses.lumenlearning.com
Phase Diagrams Chemistry Describe The Region Where A Solid-Liquid Equilibrium Exists Solid, liquid, and vapour, at certain temperatures and pressures. The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. Low temperature and high pressure favor solid. a pure substance may exist in any of the three phases: to be able to identify the triple point, the critical point, and four regions: When its temperature or. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From slideplayer.com
and Equilibrium review (Items of 200 ways ..) ppt download Describe The Region Where A Solid-Liquid Equilibrium Exists a pure substance may exist in any of the three phases: Solid, liquid, and vapour, at certain temperatures and pressures. The state exhibited by a given. Low temperature and high pressure favor solid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Solid, liquid, gas, and a supercritical fluid.. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From www.numerade.com
SOLVED 4 Consider the phase diagram in the figure below, which Describe The Region Where A Solid-Liquid Equilibrium Exists a pure substance may exist in any of the three phases: to be able to identify the triple point, the critical point, and four regions: The state exhibited by a given. Solid, liquid, gas, and a supercritical fluid. When its temperature or pressure. Solid, liquid, and vapour, at certain temperatures and pressures. Low temperature and high pressure favor. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From learncheme.com
ssleequilibrium LearnChemE Describe The Region Where A Solid-Liquid Equilibrium Exists to be able to identify the triple point, the critical point, and four regions: The state exhibited by a given. a pure substance may exist in any of the three phases: consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Solid, liquid, gas, and a supercritical fluid. When. Describe The Region Where A Solid-Liquid Equilibrium Exists.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Describe The Region Where A Solid-Liquid Equilibrium Exists to be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. a pure substance may exist in any of the three phases: Low temperature and high pressure favor solid.. Describe The Region Where A Solid-Liquid Equilibrium Exists.